
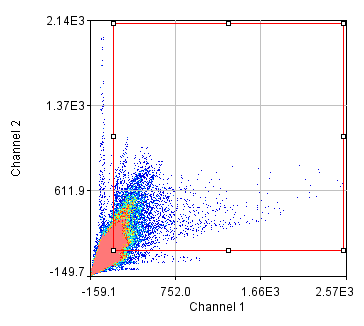
This provided improved possibilities for evaluation and visualization of colocalization. Later, the intensity-correlation based methods have been further elaborated by Li et al., who compared the pixel intensity of a detection channel with ( A i − A mean) × ( B i − B mean), where A i and B i are individual intensities of a pixel in channels A and B, while A mean and B mean are the mean intensities 5. This concept has been followed up by the same group, suggesting two coefficients M1 and M2, which describe the extent of the fluorescence of colocalizing objects relative to the total fluorescence in channel 1 and 2, respectively 4. proposed Pearson's correlation coefficient as a more quantitative measure of colocalization based on the pixel-intensity correlation of two fluorescence channels 3. The underlying statistical background had already been developed in 1896 by Pearson 2, but only about hundred years later, Manders et al. These issues had been recognized quite early in the development of fluorescence microscopy, which has led to the concept of calculating statistical parameters to evaluate the correlation of fluorescence-intensities of two (or more) detection channels on a pixel-by-pixel basis. Nevertheless, such a visual evaluation requires comparable fluorescence intensities of the two markers and is obviously far from being quantitative. A method that is still used by many scientists is to assess the color overlay of two different fluorescent markers, with for instance green and red fluorescence resulting in a yellow color in case of colocalization. In many cases, the methods to analyze and score microscopic colocalization are often simple and descriptive rather than quantitative. Furthermore, these techniques require expensive equipment that is not easily available and rather complex to use. Sophisticated novel techniques such as superresolution microscopy address these questions with better precision 1, nevertheless they can still not discriminate unambiguously between functional interaction and incidental colocalization. However, in particular the latter assumption is often an over-interpretation, as the occurrence of two molecules in the same subcellular regions does not necessarily mean that they are physically binding to each other. Since discrimination of different fluorescence colors is rather easy with standard microscopy equipment, many scientists use fluorescence colocalization as an indication that a molecule localizes to a certain compartment or that two molecules interact with each other.

Answering these questions is often essential to clarify cellular processes as they are frequently regulated by macromolecular interactions or by specific localizations of molecules within the complex compartmental structure of eukaryotic cells. This allowed addressing central issues in cell biology and life sciences, such as the question, whether a certain protein localizes to a specific subcellular compartment or whether it shares the same localization with another molecule of interest. In the last two decades, we have seen an incredible progress in fluorescence microscopy techniques and in parallel a significant advance in methods to label proteins genetically by fusing them to spectrally distinct fluorescent proteins.
COLOCALIZATION IMAGEJ SOFTWARE
We designed a macro for the Fiji-version of the software ImageJ and tested the performance systematically with various organelle markers revealing an improved robustness of our approach over classical methods. Our aim was to improve this type of analysis and we developed a novel method combining object-recognition based colocalization analysis with pixel-intensity correlation to calculate an object-corrected Pearson coefficient. We observed that these correlation coefficients are prone to false positive results and tend to show high values even for molecules that reside in different organelles.
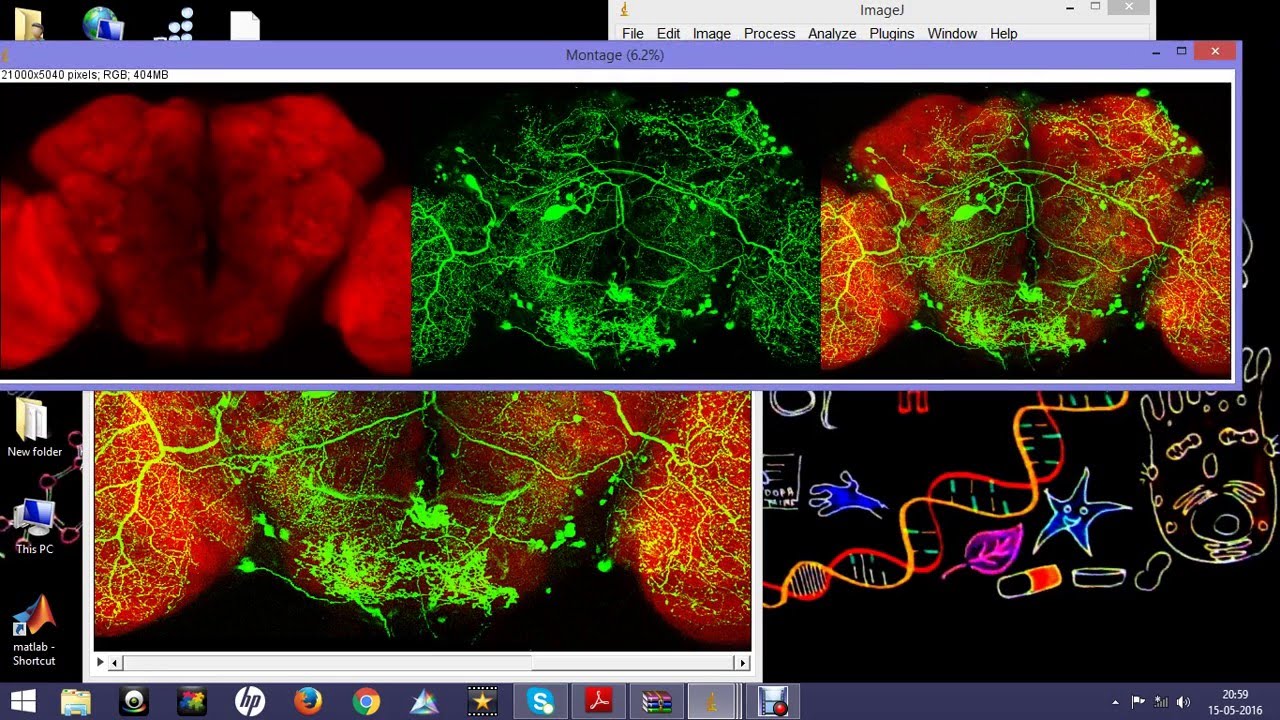
Since a mere visual estimation does not allow quantification of the degree of colocalization, different statistical methods of pixel-intensity correlation are commonly used to score it. The question whether two proteins interact with each other or whether a protein localizes to a certain region of the cell is often addressed with fluorescence microscopy and analysis of a potential colocalization of fluorescence markers.


 0 kommentar(er)
0 kommentar(er)
